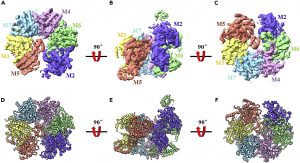
The central step in the initiation of eukaryotic DNA replication is the loading of the minichromosome maintenance 2–7 (MCM2-7) complex, the core of the replicative DNA helicase, onto chromatin at replication origin. Here, we reported the cryo-EM structure of endogenous human single hexameric MCM2-7 complex with a resolution at 4.4 Å, typically an open-ring hexamer with a gap between Mcm2 and Mcm5. Strikingly, further analysis revealed that human MCM2-7 can self-associate to form a loose double hexamer which potentially implies a novel mechanism underlying the MCM2-7 loading in eukaryote. The high-resolution cryo-EM structure of human MCM2-7 is critical for understanding the molecular mechanisms governing human DNA replication, especially the MCM2-7 chromatin loading and pre-replicative complex assembly.
Citation:
Naining Xu, Qingpeng Lin, Honglei Tian, Changdong Liu, Peiyi Wang, Ching Monica Suen, Hongyu Yang, Ye Xiang, Guang Zhu, Cryo-EM structure of human hexameric MCM2-7 complex, iScience, Volume 25, Issue 9, 2022, 104976, ISSN 2589-0042, https://doi.org/10.1016/j.isci.2022.104976.
(https://www.sciencedirect.com/science/article/pii/S2589004222012482)