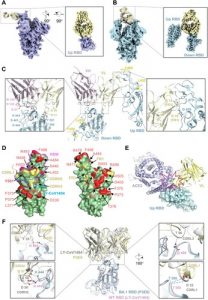
Increasing spread by SARS-CoV-2 Omicron variants challenges existing vaccines and broadly reactive neutralizing antibodies (bNAbs) against COVID-19. Here we determine the diversity, potency, breadth and structural insights of bNAbs derived from memory B cells of BNT162b2-vaccinee after homogeneous Omicron BA.1 breakthrough infection. The infection activates diverse memory B cell clonotypes for generating potent class I/II and III bNAbs with new epitopes mapped to the receptor-binding domain (RBD). The top eight bNAbs neutralize wildtype and BA.1 potently but display divergent IgH/IgL sequences and neuralization profiles against other variants of concern (VOCs). Two of them (P2D9 and P3E6) belonging to class III NAbs display comparable potency against BA.4/BA.5, although structural analysis reveals distinct modes of action. P3E6 neutralizes all variants tested through a unique bivalent interaction with two RBDs. Our findings provide new insights into hybrid immunity on BNT162b2-induced diverse memory B cells in response to Omicron breakthrough infection for generating diverse bNAbs with distinct structural basis.
Citation: Zhao, F., Lin, X., Cai, K., Jiang, Y., Ni, T., Chen, Y., Feng, J., Dang, S., Zhou, C.-Z. and Zeng, Q. (2022), Biochemical and structural characterization of the cyanophage-encoded phosphate-binding protein: implications for enhanced phosphate uptake of infected cyanobacteria. Environ Microbiol, 24: 3037-3050. https://doi.org/10.1111/1462-2920.16043